Abstract:
Back ground: Ultraviolet (UV) radiation is a known carcinogen․ The two types of UV radiation that affect the skin are known as ultraviolet A (UVA) and ultraviolet B (UVB)․ Both have been linked to skin cancer and an overall weakening of the immune system․
UVB rays mostly affect the surface of the skin and are the primary cause of sunburn․ Overexposure of UVB causes cellular damage in the basal and squamous layers of the skin, and is the main cause f basal cell and squamous cell carcinomas․
UVA rays penetrate more deeply into the skin, affecting the melanocytes and are implicated in the development of the third main type of skin cancer, melanoma․
Result: Sunscreen, also known as sun block and suntan lotion, is a lotion, spray, gel or other topical product that absorbs or reflects some of the sun’s ultraviolet (UV) radiation and thus helps protect against sunburn
Depending on the mode of action, sunscreens can be classified into physical sunscreens (i․e․, those that reflect the sunlight) or chemical sunscreens (i․e․, those that absorb the UV light)․
Sunscreen use can help prevent melanoma and squamous cell carcinoma, two types of skin cancer․ There is little evidence that it is effective in preventing basal cell carcinoma
Conclusion: Sunscreen has been proven to decrease the development of skin cancer․ It helps to prevent facial brown spots and skin discolorations․ It also helps to reduce the appearance of facial red veins and blotchiness․ It slows down the development of wrinkled, premature aging skin․
Introduction:
Bemotrizinol (INN/USAN, INCI bis-ethylhexyloxyphenol methoxyphenyl triazine) is an oil-soluble organic compound that is added to sunscreens to absorb UV rays․
Chemically, Bemotrizinol is known as: 2,4-Bis-1[4-(2-ethyl-hexyloxy)-2-hydroxy]-phenyt}-6-(4-Methoxy phenyl)- (1,3,:5)-triazine (CASRN: 187393-00-6)․
The brand name for the product is Sunbest-S (Bemotrizinol) produced by Vinner Labs Pvt Ltd․
The official International Nomenclature Cosmetic Ingredient (INCI) name for this product is: Bis-Ethylhex loxy phenol Methoxyphenol Triazine (“BEMT”)․
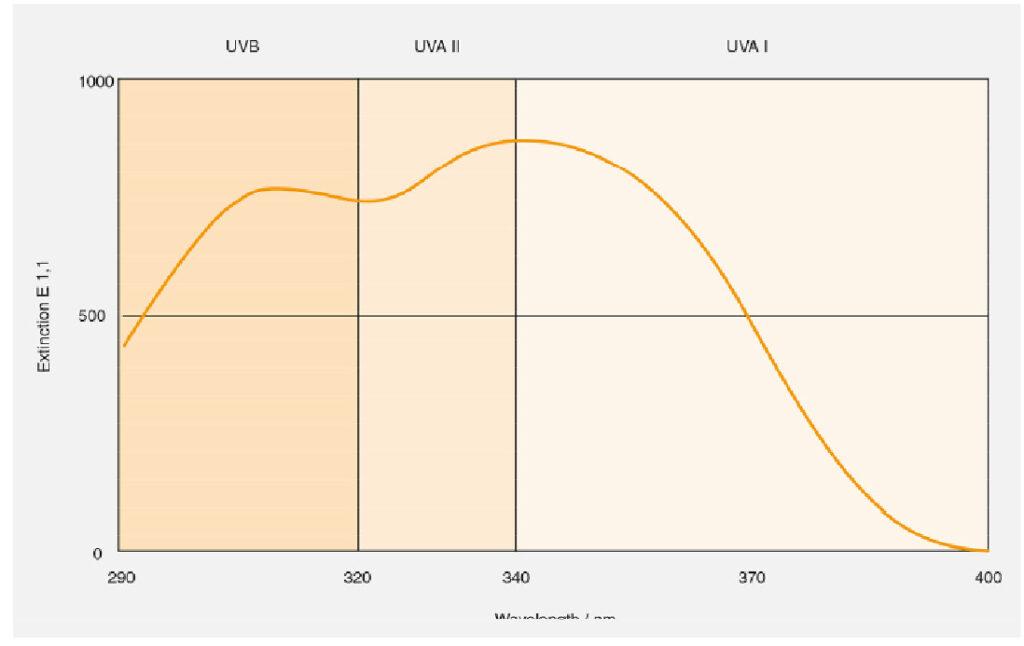
Bemotrizinol is a broad-spectrum UV absorber, absorbing UVB as well as UVA rays․ It has two absorption peaks, 310 and 340 nm․ It is highly photostable․ Even after 50 MEDs (minimal erythemal doses), 98․4% remains intact․ It helps prevent the photo degradation of other sunscreen actives․
Bemotrizinol has strong synergistic effects on the SPF when formulated with other sunscreens․ It is the most effective UV absorber available measured by SPF, based on the maximum concentration permitted by European legislation
UV spectrum of Bemotrizinol :
Analytical studies:
A novel, simple, validated stability indicating HPLC method was developed for determination of EHT and Bemotrizinol․ Stability indicating power of the method was established by forced degradation study․
The chromatographic separation was achieved with Waters X Bridge column, by using mobile phase consisting of a mixture of acetonitrile : tetrahydrofuran : water (38 : 38 : 24, v/v/v)․ The method fulfilled validation criteria and was shown to be sensitive, with limits of detection (LOD) and quantitation (LOQ) of 0․024 and 0․08 µg for EHT and 0․048 and 0․16 µg for Bemotrizinol, respectively․
The developed method is validated for parameters like precision, accuracy, linearity, solution stability, specificity, and ruggedness as per ICH norms․ Design expert with ANOVA software with linear model was applied and a 23full factorial design was employed to estimate the model coefficients and also to check the robustness of the method․
Results: The two-level full factorial design, 23with 10 runs including two-centre-point analysis based on the variance analysis (ANOVA), demonstrated that all three factors, as well as the interactions between retention time of EHT, Bemotrizinol, and USP plate count for EHT, are statistically significant․
Safety studies:
Bemotrizinol was tested phototoxicity in humans, photoallergenicity in humans Based on the tests Bemotrizinol did not cause sensitization, photo irritation, or photosensitization when applied to the skin of guinea pigs or humans․
Not genotoxic in several different assays with and without UV activation․
In an in vitro assay, exhibited low penetration (<0․1 %) across human skin․
Efficacy studies:
Bemotrizinol is the first True” broadband-tvpe UV-absorber on the market․ It provides overall protection, fully covering the UV-A and UVB spectrum in contrast to other UV filters on the market today; is indeed photostable – in contrast to other currently available UV-A filters; has a favorable safety profile; and is extremely easy to formulate in W/O or O/W emulsions․ Test datas indicates that Bemotrizinol has a much higher efficacy than what is currently available on the market․
Clinical Studies:
Primary Skin irritation study in Rabbits: Not irritating
Primary Eye Irritation study in rabbits: Minimally Irritating
Skin Sensitization: Not sensitizing
Phototoicity in Guinea pigs: Not phototoxic
Photoallergenicity in guinea pigs: Not photoallergenic
Regulatory status:
In addition to the European Union, Bemotrizinol has also been approved for use as a UV-filter in sunscreens in many other countries including key countries such as Australia, China, Korea and Taiwan․
Conclusion:
Bemotrizinol is the excellent performance as a photo stable broad-spectrum UV filter; it is compatible with organic and inorganic filters and shows synergistic effects with UVB filters for high-SPF sunscreens․ It also helps to reduce the appearance of facial red veins and blotchiness․
Bemotrizinol shows indeed a very strong synergy with the two most efficient UVB filters
Bemotrizinol, beyond its own efficiency is also an enhancer for the properties of other filters and is thus used in combination with numerous other UV filters․
References:
Arcelin,G․(1997a)․ Acute dermal toxicity study with CGF-C-1607 in rats․ RCC project 651420․ Sponsored by Ciba Chemikalien GmbH․
Arcelin,G․(1997b)․ Acute oral toxicity study with CGF-C-1607 in rats․ RCC project 651407․ Sponsored by Ciba Chemikalien GmbH․
Time and Extent Application for Bemotrizinol – UV filter
Pathak M․A․ Photoprotection Against Harmful Efffects of Solar UV-B and UV-A Radiation․ In: Lowe N․L․, Shaat N․A․ and Pathak M․A․, Sunscreens, Development, Evaluation, and Regulatory Aspects, 2”d ed․, Marcel Dekker, New York (1997) 59-79․
Research Article- Development and Validation of a Stability Indicating RP-HPLC Method for the Determination of Two Sun Protection Factors – Chinmoy Roy and Jitamanyu Chakrabarty
Disclaimer:
These details are based on information we believe to be reliable and are offered in good faith but without guarantee, as conditions and methods of use are beyond our control․
Vinner Labs P Ltd make no warranties as to the accuracy or appropriateness of the data, and recommend that prospective users determine for themselves the suitability of the materials and suggestions for use prior to their adoption․ We also recommend that approval is sought by prospective users from the respective regulatory authorities where appropriate to determine fitness for purpose․
